
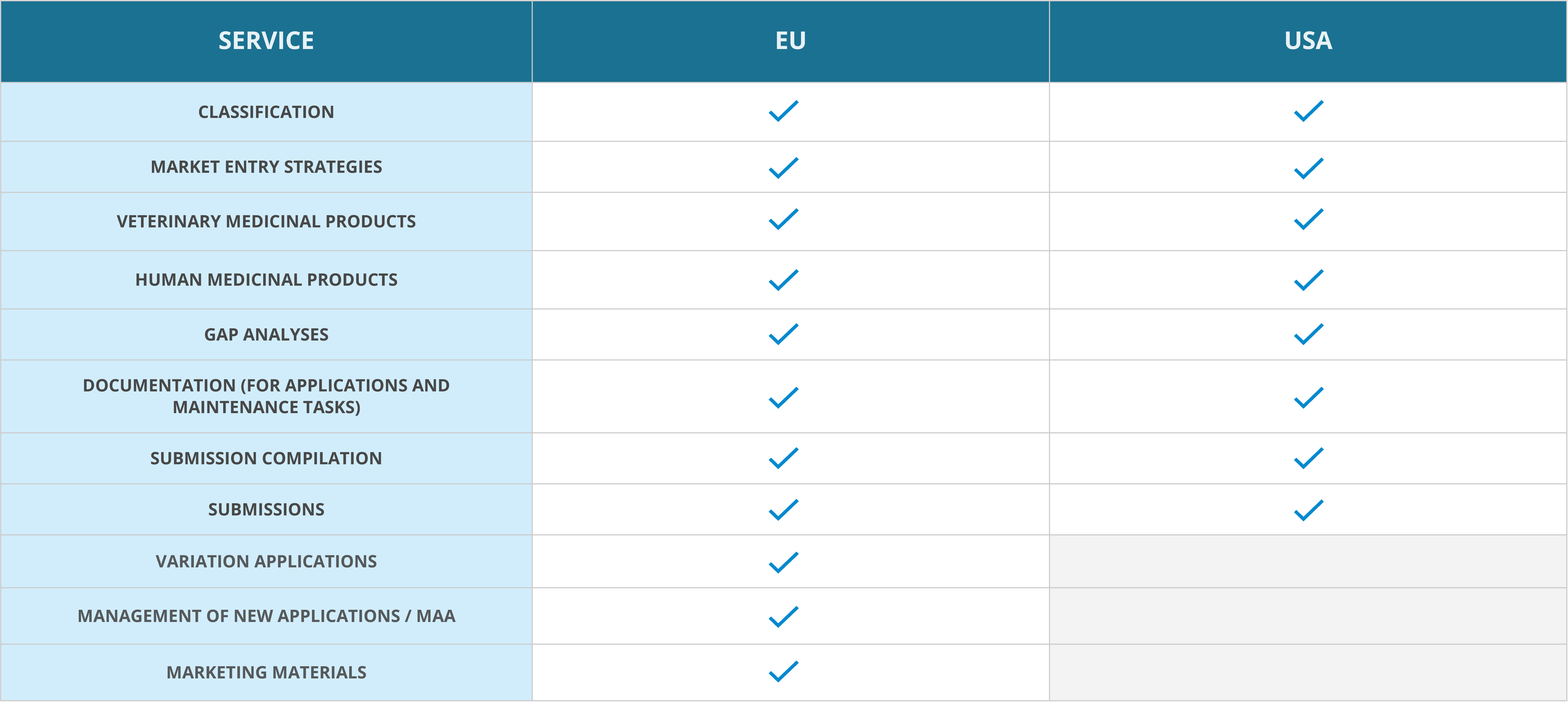
REGULATORY AFFAIRS services for HUMAN AND VETERINARY MEDICINES in the EU and the USA
Medfiles provides comprehensive regulatory affairs services in the EU and can support also in regulatory affairs for US. Our experts work in Finland, Estonia, Latvia and Lithuania and manage regulatory affairs with any EU authority. With our qualified partner network, we can handle the national special characteristics in all EU countries. We help our clients with documents related to new drug applications and maintenance tasks also in the USA.
When you need regulatory affairs expertise, we are happy to help you with comprehensive headquarters level services or take care of your marketing authorisation applications and maintenance activities as your affiliate. We are there for you in all stages of the pharmaceutical product lifecycle, from product development to marketing authorisation application, registration process and marketing authorisation maintenance – or we can help in specific tasks, whatever your need is.
By choosing to work with us, you can be sure that your medicinal product meets the authority requirements. Our Regulatory Affairs Team of some 40 highly qualified experts has extensive professional experience in the pharmaceutical sector and working with regulatory authorities. To ensure the high quality of our services, our experts undergo regular training and are always up to date with the latest requirements and practices.

Free WEBINAR: EU Veterinary Medicinal Products Regulation from the perspective of variations and the quality part of the pharmacovigilance system
In our webinar you will learn about the main changes and requirements brought about by the Veterinary Medicinal Products Regulation. In the webinar we will discuss variation management and the relationship between pharmacovigilance systems and the related quality measures.
Regulatory affairs services

MARKETING AUTHORISATION SERVICES FROM STRATEGY TO APPLICATION AND LAUNCH
Our experts are here to help in planning the strategy for your marketing authorisation application (MAA) already at the beginning of the product development stage. At this stage, it is important to determine whether a new product can be classified as a medicine or not. If the product is classified as a medicinal product, the legal basis of its marketing authorisation application determines whether the product’s efficacy and safety can be demonstrated based on literature, or whether non-clinical or clinical trials are required. It is also smart to get acquainted early on in the process with similar medicinal products that may already be authorised in the target countries.
CAREFUL PROJECT PLANNING ENSURES SMOOTH MARKETING AUTHORISATION PROCESS
Medfiles’ highly qualified and competent team of experts has on average more than 10 years of experience in marketing authorisations, working both for the pharmaceutical industry and at the regulatory authorities. By providing continuous training to our experts and constantly keeping up to date with changes in legislation, we guarantee our clients expert service that is always in line with the latest regulatory requirements. And when needed, we utilise our reliable and qualified partner network to provide a wider range of services for our clients.
We can take care of the entire marketing authorisation application process (DCP/MRP/CP/NP) for you, in any EU country, from registration strategy to marketing authorisation. You can also outsource only parts of your process to us. Each client project is appointed a dedicated project manager and a project team. By providing support for product classification and scientific advice throughout the application process, we will guide you from research to market.
In the EU, we will help you with
- creating registration strategies
- product classifications: drugs, medical devices and food supplements
- GAP analyses of marketing authorisation dossiers
- preparation and submission of marketing authorisation applications
- coordinating application procedures.
In the United States, we will help you with
- planning of market expansion to the US
- documentation (new drug applications and supplemental applications)
- market entry strategies
- product classifications.
We also offer comprehensive training on new marketing authorisation applications and marketing authorisation strategies.
We have assisted our clients in more than 100 marketing authorisation processes. Our experience with different types of marketing authorisation procedures:
- 55 % DCP (decentralised procedure)
- 24 % MRP (mutual recognition procedure)
- 17 % NP (national procedure)
- 4 % CP (centralised procedure)
We can also help with market access activities:
- price and reimbursement status applications
- marketing authorisation renewal applications
- price comparisons and price level determinations
- reference price declarations.
See also:
- Global pharma company & Medfiles: Cooperation in obtaining and maintaining marketing authorisations in the EU
- Marketing authorisation application processes efficiently under one roof – for all EU countries
- Centralised marketing authorisation process requires diverse expertise – Medfiles’ expertise employed as the driving force for a new veterinary MA

MARKETING AUTHORISATION MAINTENANCE SERVICES ENSURE THAT YOUR MARKETING AUTHORISATION IS UP TO DATE
Competent marketing authorisation maintenance services ensure that your medicinal product remains compliant and available on the market. Our experienced Marketing Authorisation Team provides comprehensive marketing authorisation maintenance services for human and veterinary medicinal products, regardless of the active substance. You can outsource the full maintenance of your product or only specific parts to us. Our team of highly competent experts with diverse scientific backgrounds specialises in the maintenance of marketing authorisations in Europe and gladly provides consultation for expanding your business and bringing your product on the market in the United States.
Our team has special expertise in local requirements in the Nordic and Baltic countries. Our experts can work either from the Medfiles office or at the client’s premises.
You can outsource to us
In the EU
- variation applications, notifications and renewal applications required by the authorities
- switches from prescription drug to over-the-counter drug status (Rx/OTC)
- marketing authorisation transfers to new marketing authorisation holders
- review of marketing materials
- training on the maintenance of marketing authorisations.
In the US
- support for market expansion to the US
- documentation (supplemental applications).
In addition to the services listed above, our experts can serve as part-time or full-time outsourced resource when you face sudden resource challenges. Our outsourcing service allows you to fix your staff shortage or get a replacement to fill in for longer absences.
See also:
Regulatory affairs services for veterinary medicines in the EU, UK and USA
Medfiles offers regulatory affairs services also for veterinary medicinal products. Our Veterinary Medicines Team includes a number of experienced specialists who are well versed in veterinary legislation and applicable regulatory guidance, such as the EU Veterinary Medicinal Products Regulation, national UK legislation and VMD guidance, as well as the US FDA guidance.
We have more than 10 years of experience in registering veterinary medicines. We keep our skills up to date by continuously following changes in industry guidelines and participating in regulatory training organised by relevant organisations, such as the EMA, FDA, MPA, Fimea and VMD. We also offer training on veterinary legislation and registrations for our clients – ask for training tailored to your team or company!
Our competent and dedicated Veterinary Medicines Team can help you with the following tasks:
In the EU and UK
- classification as a veterinary medicinal product (veterinary medicinal product vs. medical device, veterinary medicinal product vs. feed or supplement)
- marketing authorisation strategies
- new marketing authorisations (NP, DCP, SRP and CP procedures)
- new vet ASMF submissions
- compilation and submission of VNeeS in the EU and UK (VMDS, the EMA gateway and CESP)
- documentation such as Part 1, Part 1c1, Part 2/Module 3
- drafting, updating and translating product information for veterinary medicines (including QRD updates to version 9.0)
- national requirements and proof-reading of packaging materials for veterinary medicines
- registration tasks at product launch: national requirements, packaging materials, updating national databases and review of marketing materials
- maintenance of marketing authorisations (VRA, VNRA, UPD and implementations)
- managing parallel processes and variations with multiple authorities.
In the USA
- classification as an animal drug and market-entry strategies
- GAP analyses of marketing authorisation dossiers (EU vs USA)
- documentation for new animal drug applications (ANADA, NADA) and supplemental applications, including labelling documents.
In addition to the above services, our regulatory affairs experts can work for you as a part-time or full-time outsourced resource. You can outsource all or part of your project to us – our team is always at your service.
Our Veterinary Medicines Team works in close collaboration with the other Medfiles units. This means that you can, for instance, outsource your veterinary pharmacovigilance to us, or benefit from our feed and feed additives services, from product development to regulatory reviews, registrations and marketing, provided by our dedicated Feed Team.
See also:
- Bimeda & Medfiles: Partnership in veterinary regulatory affairs in the EU
- Best practices for QRD updates of PI texts of veterinary medicines
- Centralised marketing authorisation process requires diverse expertise – Medfiles’ expertise employed as the driving force for a new veterinary MA

Free WEBINAR: EU Veterinary Medicinal Products Regulation from the perspective of variations and the quality part of the pharmacovigilance system
In our webinar you will learn about the main changes and requirements brought about by the Veterinary Medicinal Products Regulation. In the webinar we will discuss variation management and the relationship between pharmacovigilance systems and the related quality measures.

PHARMACEUTICAL-CHEMICAL (CMC) EXPERT SERVICES AND CMC CONSULTING
Understanding the requirements for chemistry, manufacturing and controls, or CMC for short, contributes to the fastest possible access of the medicinal product to patients. Medfiles offers a wide range of professional services related to CMC consultation and documentation – our competent team of experts takes into account the existing requirements, writes marketing authorisation documentation and helps avoid unnecessary delays during product development and registration.
Our professional team undergoes constant training to keep up with the latest guidelines. Our extensive work experience in the pharmaceutical industry and with regulatory agencies ensures efficient high-quality services. Our service comes with guaranteed back-ups for our experts, regardless of the duration of the project. We take care of the documentation work for you, both for new marketing authorisation applications and individual variation applications – ready for submission to both European pharmaceutical authorities and the US FDA. Our CMC consulting and CMC documentation services are tailored to each client’s needs and can be integrated in larger-scale service packages that include Medfiles’ registration services, product development and analytical services, clinical research services or medical device services.
Our pharmaceutical-chemical documentation service covers the following areas:
- Marketing authorisation applications
- documentation for module 3 or Part II
- quality overall summaries
- requests for additional information during marketing authorisation procedures
- evaluation of dossiers (gap analysis)
- Variation applications
- consultation and classification of variations
- preparation of documentation
- Clinical trial application documentation (IMPD, IND)
- Active substance documentation (ASMF, DMF and CEP)
- Validation plans and reports
- Risk assessments (nitrosamines, elemental impurities)
- Consultation and training
PROJECT MANAGEMENT, DRUG DEVELOPMENT AND TECHNOLOGY TRANSFERS
Our experts in the CMC Team have extensive work experience in both analytical chemistry and pharmaceutical manufacturing. We have assisted our clients with several development projects and technology transfers. Our CMC experts are experienced in coordinating analytical method transfers and consulting in analytical chemistry.
Our CMC services include:
- outsourced experts for your projects – for both manufacturing processes and analytics
- competent coordination of technology transfers, taking into account the regulatory requirements
- compilation of quality documentation to support smooth regulatory approval of technology transfers.
“Medfiles’ expert provided us invaluable support in solving an analytical problem. Thank you for your hard work – it really exceeded our expectations!”
– Client feedback in 2023
“In particular, Medfiles’ very strong CMC expertise has been helpful in performing gap evaluation on existing files, addressing gaps, and preparing updated and upgraded dossiers for submissions in Europe.”
– Bimeda Animal Health Limited
See also:

ELECTRONIC submission services (ESUBMISSION)
Electronic common technical documents, better known as eCTDs, are needed for electronic submissions, along with eCTD publishing tools. The annual expenses of these tools can be significant in relation to the actual need for the tool, especially for small businesses. In such cases, it may be wise to save resources and outsource your eCTD publishing tasks. Medfiles uses an eCTD publishing tool that is widely used by various authorities around the world. With over 10 years of experience in eCTD publishing, Medfiles is a reliable and competent partner for all your eCTD publication activities.
We compile electronic applications in accordance with existing regulations and guidelines. In case of eCTD or VNeeS, we always use the current version of the appropriate validation tool to ensure that the compiled sequence is technically valid, and send it to the authorities using the designated channels, such as CESP or EMA Gateway.
Our team is experienced with all eSubmission formats and can consult other medical or pharmaceutical experts, ensuring the high quality of our eSubmission services. A second expert review is always included in our eSubmission price.
Our services include:
- compilation of eCTD and VNEES sequences and other types of electronic applications
- submission of applications via the CESP portal, EMA Gateway, Eudralink, FDA ESG or secure email.
See also:

PHARMACEUTICAL PRODUCT INFORMATION AND TRANSLATION SERVICES
Our Product Information Team consists of translators and regulatory experts who have years of experience in tasks related to product information texts: summaries of product characteristics (SmPC), package leaflets (PIL) and labelling. Our competent team delivers easy to read, compliant texts tailored to the target audience, even within strict schedules. In the translation process, we utilise computer-assisted translation tools and translation memory tools to ensure the consistency of style and terminology. Regulatory review is included in our process, and we employ the four-eyes principle to guarantee the highest possible quality.
Our services include:
- preparation of product information text
- translations of product information texts
- in Finnish, Swedish, English, Estonian, Latvian and Lithuanian
- other languages through our partner network
- package leaflet user tests and bridging reports
- proofreading of mock-ups
- preparation and updating of abbreviated SmPCs
- updating local authority databases.

REVIEW OF MARKETING MATERIALS FOR MEDICINAL PRODUCTS
When you need to ensure that your marketing materials for medicinal products are compliant, our marketing material experts are glad to help. Our experienced team will make sure that your materials comply with the applicable local regulations and marketing regulations based on the classification of your product (Rx/OTC). Our service includes medical, editorial and ethical review. After our review process, you can be sure that the medical facts, language, claims and references in your marketing materials are in accordance with the local legislation and ethical codes, without losing the marketing edge.
Our experts have extensive experience in various types of marketing materials and working with businesses of all sizes, from small pharmaceutical companies to large-scale multinational enterprises. We have local expertise in the Nordic and the Baltic countries, and our team reviews materials according to the local requirements. We give clear feedback for your materials, evaluate the potential risks and offer solutions to overcome them.
We will be happy to review the following materials for you:
- Over-the-counter drug brochures, social media content, websites and promotional videos
- Prescription drug brochures and other materials
- Business PowerPoint presentations and other presentation materials
- Company invitation letters
We also offer tailor-made training on the subject.

GOOD DISTRIBUTION PRACTICE (GDP), RESPONSIBLE PHARMACIST AND PRODUCT COMPLAINTS
Medfiles offers responsible pharmacist services for medicinal product wholesalers in Finland. Our team of qualified responsible pharmacists for wholesalers have suitable degrees in pharmacy, the required training in the GDP, the required competence and experience as well as extensive knowledge of the pharmaceutical industry. We offer full responsible pharmacist services or tailor-made solutions according to your needs – depending on whether you need a full-time solution or a short-term back-up, for example during holidays. Our team also has extensive knowledge of product complaint handling and can assist you with other GDP-related tasks.
Our service package can be customised to suit your company’s needs. We offer the following services:
Responsible pharmacists in Finland
- Full-time designated person
- Deputy responsible pharmacist
- Delegated tasks
GDP services
- Wholesale dealer’s authorisation application
- Preparation and update of standard operating instructions (SOP)
- GDP audits
- GDP training and consultancy
- Consulting services
- import and logistics
- narcotics control and mandatory reserve supplies
- Handling product complaints
Our services are always tailored to best meet your needs and objectives. Contact us and ask for a quote on our regulatory affairs services.
Free guide: What you need to know about active substance registration strategy?
Drug substance quality must be demonstrated in the marketing authorisation application, and in Europe, there are three different ways to do this. The choice depends mainly on the API in question. We have created a guide on the basic information you need to know about registration, so that you can make the best choices for your project.
Why outsource your regulatory affairs tasks to Medfiles?
Our Regulatory Affairs Team has extensive experience in human and veterinary medicinal products, sterile and non-sterile products and biological medicinal products. Our team of 40 experts consists of specialists with educational backgrounds in pharmacy, natural sciences and chemical engineering.
We are ready to help you with a wide range of regulatory tasks related to your product’s lifecycle, whether your company is a small or medium-sized business, a large enterprise or an academic research organisation.
Our experts are happy to help:
- non-EU companies that need expertise in the pharmaceutical legislation in the EU
- companies that have not previously had products classified as medicinal products in their product portfolio
- companies that want to outsource routine tasks and prioritise their time for something else, such as new product development projects
- companies facing surprising or challenging changes in their resources. Ask for a part-time or full-time expert, for short- or long-term outsourcing.
- companies with products in many different product categories: pharmaceuticals, medical devices, IVDs, food supplements and cosmetics. We have highly qualified and experienced teams for each of the different product categories. In case of borderline products, our teams work closely together to help you select the right registration route.
- companies with projects that require broad expertise: our service portfolio includes clinical trial services, product development and laboratory services, regulatory and pharmacovigilance services and translation services – all under one roof.
Over 30 years of experience in regulatory affairs
100+
marketing authorisation processes
1600+
WRITTEN VARIATIONS / RENEWALS
1500+
REGULATORY SUBMISSIONS / YEAR
200+
PROMOTIONAL MATERIAL REVIEWS / YEAR
Contact us
You may also be interested in:
- Free guide: What you need to know about active substance registration strategy?
- Marketing authorisation application processes efficiently under one roof – for all EU countries
- Free guide: Determining storage conditions for pharmaceuticals
- Best practices for analytical method transfers
- Free webinar: EU Veterinary Medicinal Products Regulation from the perspective of variations and the quality part of the pharmacovigilance system
- Bimeda & Medfiles: Partnership in veterinary regulatory affairs in the EU
- Centralised marketing authorisation process requires diverse expertise – Medfiles’ expertise employed as the driving force for a new veterinary MA
- Best practices for QRD updates of PI texts of veterinary medicines
- A team of 12 experts ensures high-quality regulatory services in the Baltic countries
Medfiles is a member of the regulanet® the Network of Regulatory Affairs consultants with members in 90 countries throughout the world.

