Medical device and IVD services ensure regulatory compliance throughout device lifecycle
Medfiles offers comprehensive expert services for medical devices and in vitro diagnostic (IVD) medical devices that cover the entire product lifecycle, from establishing a quality management system (QMS) to post-market surveillance (PMS). Utilising our expertise in your product development projects ensures that all regulatory requirements are taken into account from the very beginning. Our team will guide you through the CE marking process and help you prepare the required documentation. If your product is already on the market, you can outsource any post-market duties to us.
Our experts can help you in various tasks throughout the product lifecycle, whether you want to place your device on the market in the European Union or outside the EU. We are here for you if you want to CE mark your device in compliance with the Medical Device Regulation (EU) 2017/745 or the In Vitro Diagnostic Regulation (EU) 2017/746, or register your product in countries outside the EU, which in the USA may require expertise related to the FDA 21 CFR Part 820 and 510(k).
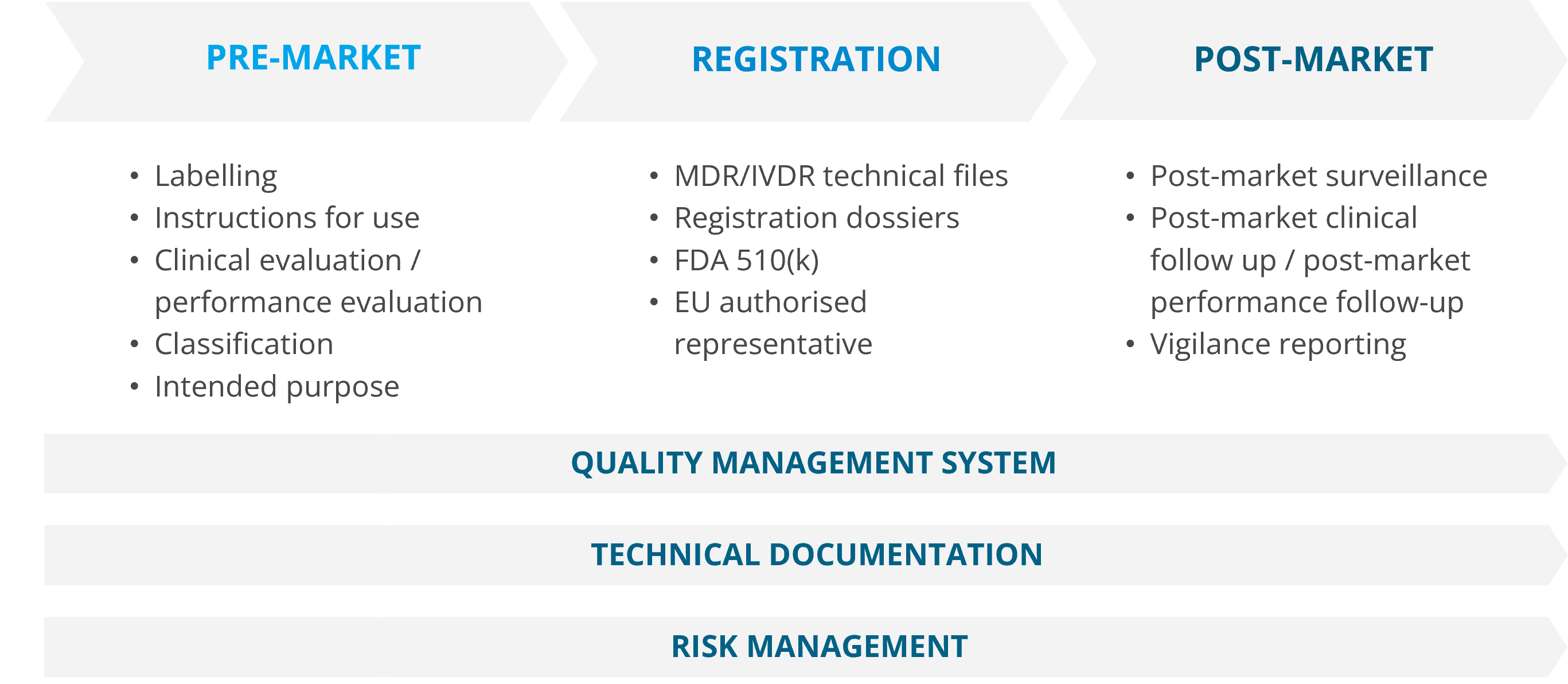
Our services cover quality management, technical documentation, clinical evaluations, performance evaluations, product registrations, post-market surveillance (PMS) and vigilance as well as other additional services, such as tailored training in our areas of expertise. If you want to make sure that your product, process or system is compliant with the relevant standards, such as ISO 13485, ISO 14971, ISO 10993, IEC 60601 and IEC 62304, our experts are at your service.
Because Medfiles has dedicated teams for medicines, clinical studies and laboratory services, our Medical Device and IVD Team has direct access to a wide range of specialist expertise in other fields – this allows us to provide comprehensive advice, for instance, for substance-based devices and combination devices.
Medical device and IVD services

Quality management system according to ISO 13485
Most major markets require implementation and maintenance of a quality management system (QMS). With our help, you can meet the specific QMS requirements in different markets around the world. ISO 13485 is an international quality management system standard for medical devices that has been developed to meet the specific needs of the industry. Several markets, however, have their own specific requirements.
Let us help you in establishing and implementing the requirements in your market areas into an efficient QMS system by:
- Providing an ISO 13485-based QMS package
- Consulting on requirements, establishment and implementation of quality management system
- Performing quality management system pre-audits, audits and gap analyses
- SOP writing, review and consultation.
You may also outsource the tasks of quality manager or quality specialist to us, including the following:
- Duties of person responsible for regulatory compliance (PRRC)
- Coordination of internal audit programs and conducting internal audits
- Coordination of supplier management and conducting vendor and supplier audits
- Coordination of post-market surveillance activities
- Coordination and performance of risk management activities.

Technical documentation for medical devices and IVDs
Medfiles offers a wide range of services related to the technical documentation of medical devices and IVD medical devices. Whether you need to draw up technical documentation for a new device or bridge the gap between the existing documentation and regulatory requirements, or you have specific questions regarding the technical documentation, we are happy to help.
If you want to CE mark your medical device and place it on the market in the EU, the technical documentation must comply with the applicable requirements in the Medical Device Regulation (EU) 2017/745 (MDR) or the In Vitro Diagnostic Regulation (EU) 2017/746 (IVDR).
Other markets outside the EU have their own requirements for the device’s technical documentation. We can assist you in compiling and managing the technical documentation of your medical device or IVD medical device also outside the EU.
To make sure your documentation will comply with the applicable requirements, we can perform a gap analysis between your existing technical documentation and requirements of the MDR/IVDR in the EU or other applicable requirements in the markets outside the EU. We can also assist you in drawing up the technical documentation for your device, including the following:
- device description and specifications
- labelling
- packaging and instructions for use
- general safety and performance requirements (GSPR) checklist
- risk management documentation
- documentation related to product verification and validation
- technical documentation on post-market surveillance (PMS).
You can ask us specific questions related to the technical documentation of medical devices and IVD medical devices, such as questions concerning the structure of the technical documentation or the requirements of a specific area of the technical documentation, such as risk management. If you need a review for your technical documentation, we can make sure it meets the regulatory requirements of the MDR/IVDR or the applicable legislations in the markets outside the EU.

Clinical evaluations for medical devices and performance evaluations for IVDs
Clinical evaluation or performance evaluation is an essential part of the conformity assessment of medical devices and IVD medical devices and mandatory according to the applicable EU regulations: the Medical Device Regulation (EU) 2017/745 (MDR) and the In Vitro Diagnostic Regulation (EU) 2017/746 (IVDR). Our experts can help you to meet these clinical evaluation and performance requirements.
In the recent years, the bar has been rising for clinical data requirements both in the EU and outside the EU, and the question of whether there is enough clinical data available from equivalent products or if clinical investigations are needed is of critical importance. No matter if you plan to tackle the requirements with review of published literature or clinical investigations of your own, we can provide the expertise and resources in:
- Creating product-specific strategies to meet clinical data requirements
- Writing clinical evaluation and performance evaluation plans and reports
- Planning, performing and reporting systematic literature reviews
- Clinical investigations and performance studies
- Writing post-market clinical follow-up (PMCF) and post-market performance follow-up (PMPF) plans and reports and assisting in PMCF/PMPF activities

Medical device and IVD product registrations
Place your medical device or IVD medical device on the market successfully around the world with the help of our services. By utilising our team’s extensive experience in your product development projects, you can be sure that all regulatory requirements are considered early on from the start. We can make sure that your product is compliant with the regulatory requirements in various markets, both in the EU and outside of the EU.
Our team is experienced in successful product registrations in cooperation with local representatives in several markets around the world. Whether you want to outsource the whole registration process or need answers to specific questions about product registration in the different markets, let us help you.
When you need to determine the intended purpose of your product, create a regulatory strategy or select suitable procedures for the conformity assessments for your product, you can rely on our expertise. We can also assist you in product classification, including borderline products and drug-device combination products, and switching product classification for example from a medicinal product to a medical device.
Wherever you intend to sell your medical device or IVD medical device, we can compile the specific registration documents needed for each market around the world. If you plan to place your product on the market in the EU, we can help with CE-marking your product according to the MDR/IVDR, and when you aim for the market in the USA, we can assist you in the FDA 510(k) process and compliance with the 21 CFR Part 820.
You can ask us specific questions about product registration in the different markets around the world, and we can help you by keeping an up-to-date list about the different laws, guidelines and standards about medical devices and IVD medical devices for any country you need. Once your product is on the market, you can outsource your maintenance tasks to us. Medfiles also provides comprehensive authorised representative services according to the MDR and IVDR requirements for device manufacturers from outside of the EU.

Post-market surveillance and vigilance services for medical devices and IVDs
Do you need help in creating a post-market surveillance (PMS) system that meets all applicable regulatory requirements? Our experts can support you in establishing and developing your post-market processes or if you need an extra pair of hands, you can outsource parts of your process to us.
Our services include:
- Consultation on post-market surveillance (PMS), post-market clinical follow-up (PMCF) and post-market performance follow-up (PMPF)
- Consultation on vigilance procedures
- Coordination of post-market surveillance activities
- Specific tasks related to PMS, PMCF and PMPF (such as systematic literature reviews and vigilance database searches)
- Incident and field safety corrective action processing and reporting

Training on requirements and regulatory environment for medical devices and IVDs
Continuous competence development is essential in a rapidly changing operating environment. Medfiles provides high-quality and timely training for companies in the MD and IVD industries. Ask for training tailored specifically to your team or company.
Possible topics could be for example:
- CE marking process of medical devices – requirements and best practices
- Medical Device Regulation 2017/745 and In Vitro Diagnostic Regulation 2017/746
- EN ISO 13485:2016
- 21 CFR Part 820 (QSR)
- Support and coaching for your quality or regulatory affairs personnel.
We will be happy to make you an offer on the desired topic.
Other services for medical devices and IVDs
With our comprehensive service portfolio in human and veterinary medicines, medical devices, in vitro diagnostic devices and food & feed and cosmetic products, our highly-educated and experienced experts are ready to share their expertise across unit boundaries – for the good of our clients.
Medfiles can also help you with:
- Translations
- The team translates different kinds of texts:
- instructions for use, labelling texts and package labels
- information leaflets, informed consents and communications with authorities and ethics committees
- website texts, marketing materials and product materials for healthcare professionals
- Our in-house working languages are English, Finnish, Swedish, Estonian, Latvian and Lithuanian, and with the help of our hand-picked partner network, we can deliver texts in many other languages.
- The team translates different kinds of texts:
- Laboratory services and R&D (drug-like devices):
Free Guide: HOW TO MEET MDR GOOD CLINICAL PRACTICE REQUIREMENTS IN PRACTICE?
Do you work with medical devices and need information on the MDR/ISO 14155:2020 good clinical practice requirements? The MDR states that clinical investigations must be performed according to good clinical practice. In addition to observing the requirements set in the MDR itself, sponsors must also be aware of the requirements set in the ISO standard. We have created a guide on what you need to know about these requirements regarding the clinical trial set-up, monitoring and ending phase.
team leader of Medical device and IVD services

Eric Schwandt
Head of Operations, Medical Device
Eric Schwandt joined Medfiles in 2022 and leads the Medical Device and IVD Regulatory Team. Eric has a M.Sc. in Oral Biology, focusing on the molecular biology of cancer.
For more than 20 years before joining Medfiles, he has held a number of regulatory affairs, QMS management, and marketing positions in the medical device and IVD industry. This has led to particular experience and interest in autoimmune disease diagnostics, POC testing, immunohistochemistry, and radiation and laser product safety.
